CONTENT: HEAT CAPACITY AND SPECIFIC HEAT CAPACITY AND VERIFICATIONS
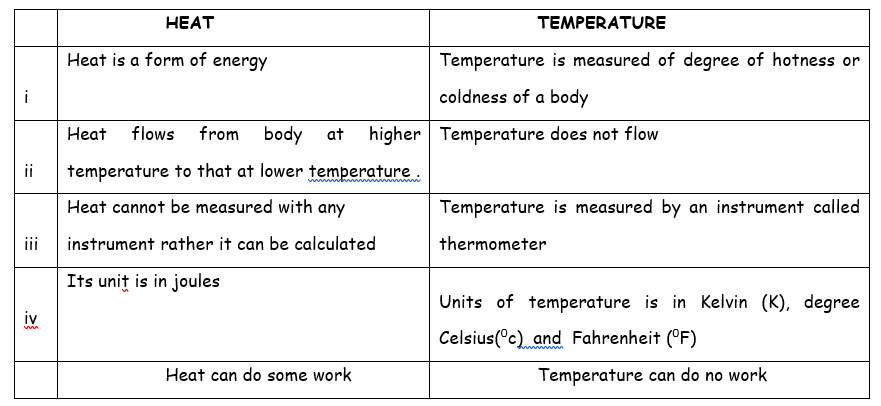
Definition of heat, heat capacity and specific heat capacity
Determination specific heat capacity
MEASUREMENT OF HEAT ENERGY
In order to assess the quantity of heat energy possessed by a body, three quantities are needed. They are:
(i) the change in temperature (∆0)
(ii) the specific heat capacity of the body (C)
(iii) mass of the body (m)
The quantity of heat( Q) of a body is a product of the three quantities above as expressed by the equation.
Q = MC∆0. It is measured in Joules
HEAT CAPACITY
This is the quantity of heat required to raise the temperature of a substance by one degree. It is measured in Joules/0K. H = mC
SPECIFIC HEAT CAPACITY OF MATERIAL
It has been discovered that the quantity of heat (Q) received by a body is directly proportional to its mass(m), change in temperature (θ2 – θ1) and also on nature of the material of which the body is made up of, stating this mathematically we can write
Q α m(θ2 – θ1)
Or
Q = mc(θ2 – θ1)................................................................(1.1)
Where c is the proportionality constant which depends on the material of which the body is made up of, c is called the specific heat capacity of the body.
From equation 1.1 above, we can define c as
Q
m(θ2 – θ1) ...........................................(1.2)
unit of c is Jkg-1K-1. (Because unit of mass is kilogram(kg), temperature in Kelvin (K).
The specific heat capacity of a substance is the quantity of heat required to raised the temperature of unit mass (1kg) of a substance through a degree rise in temperature (i.e 10 C or 1K)
Specific heat capacity of water is 4200JKg-1 K-1 ( or 4.2Jg-1 K-1 when the mass is in grams), it is observed that the specific heat capacity of water is higher than that of most other substances.
Specific heat capacities of some substances are given intable below
substance C in Jkg-1 K-1
Lead 130
Mercury 140
Brass 380
Zink 380
Copper 400
Iron 450
Glass 670
Aluminium 900
Ice 2100
Methylated spirit 2400
Sea-water 3900
Water 4200
https://youtu.be/_L9IQW6oaQE
THE HEAT CAPACITY(C)
When an entire mass of a body is taken into consideration instead of a unit mass, we talk of heat capacity. When the temperature is raised by 1K that is θ2 – θ1 is unit, then quantity of heat C is given by C = mc, this is called the heat capacity C.
The heat capacity of a body is the quantity of heat required to raise the temperature ot the entire body through one degree rise in temperature (1K).
Unit of C is in Joule per Kelvin (JK-1)
THE HEAT CAPACITY(C)
When an entire mass of a body is taken into consideration instead of a unit mass, we talk of heat capacity. When the temperature is raised by 1K that is θ2 – θ1 is unit, then quantity of heat C is given by C = mc, this is called the heat capacity C.
The heat capacity of a body is the quantity of heat required to raise the temperature ot the entire body through one degree rise in temperature (1K).
Unit of C is in Joule per Kelvin (JK-1)
https://youtu.be/TqJFIBODrjM
EXAMPLE 1
Calculate the mass of iron with heat capacity of 900 Jkg -1 K-1
SOLUTION
Specific heat capacity of iron = 450Jkg-1 K-1 using heat capacity C = mc and substituting yields:
900 = m x 450
m = 900/450
= 2.0kg
Example 2
What quantity of heat is needed to raise the temperature of 20kg of aluminium through 10k ?
Solution
Specific heat capacity of aluminium is 900Jkg -1 K -1
Using Q = mc (θ2 - θ1) and substituting we get,
Q = 20 x 900 x 10
= 180000J
= 18 x 104 J.
EXAMPLE 3
What is the heat capacity of 20kg of brass?
Solution
Specific heat capacity of brass is 380Jkg -1K -1
C = mc = 20 x 380
= 7600Jk-1
SPECIFIC HEAT CAPACITY
Specific heat capacity ( c )of a substance is the heat required to raise the temperature of a unit 0r 1Kg mass of the substance through one degree.
The quantity of heat Q received by a body is proportional to its mass (m), and temperature change
(02 - 01) and on the nature of the material the body is made of.
This Q α m (02 - 01)
Q = mC (02 - 01)
C is a constant of proportionality called the specific heat capacity of the body, which depends on the nature of the body.

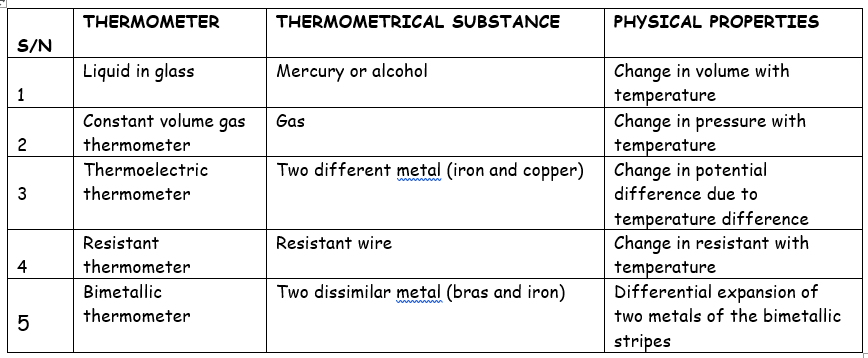
specific heat capacity of a body can be determined by using
(i) the method of mixtures
(ii) the electrical method
DETERMINATION OF SPECIFIC HEAT CAPACITY BY METHOD OF MIXTURES.
The solid lead block is weighed on a balance to be ms. A lagged calorimeter is dried and weighed to be mc. It is then reweighed to obtain the mass of water m w ,when half filled with water. The initial temperature of the water is taken to be 01.
The lead block is suspended in boiling water with a temperature 03 after which it is transferred to the calorimeter and the mixture stirred to maintain a uniform temperature 02.
The specific heat capacity of the lead can be calculated using the fact that heat loss by the lead = heat gained by calorimeter and water. Given the specific heat capacity of calorimeter and water to be C[sub]c[/sub] and C[sub]w[/sub] respectively.
 https://youtu.be/yhNHJ7WdT8A
DETERMINATION OF SPECIFIC HEAT CAPACITY BY ELECTRICAL METHOD.
https://youtu.be/yhNHJ7WdT8A
DETERMINATION OF SPECIFIC HEAT CAPACITY BY ELECTRICAL METHOD.
To calculate the specific heat capacity Cb of a solid brass block, we make two holes in a weighed brass block into which a thermometer and a heating element connected to a source of power supply are inserted. Oil is poured in the holes to ensure thermal conductivity.
Assuming no heat is lost to the surrounding, the total amount of electrical heat energy supplied by the coil IVt = heat gained by the brass, MC[sub]b[/sub]0
Ivt = MC[sub]b[/sub]0……………(1)
From v = IR (ohms law)
I2 Rt = MC[sub]b[/sub]0………….(2)
V2 t = MC[sub]b[/sub]0………(3)
R
 https://youtu.be/HAPmwu7byGM
EXAMPLE 1
https://youtu.be/HAPmwu7byGM
EXAMPLE 1
250g of lead at 170ºC is dropped into 100g of water at 0ºC. If the final steady temperature is 12ºC, calculate the specific heat capacity of lead.
(Cw = 4.2 x 103 J/kgk)
heat lost lead = heat gained by water
0.25 x c x (170 – 12) = 0.1 x 4200 (12 – 0)
C = [sup]420 x 12[/sup]/[sub]0.25 x 158[/sub]
C = 127.6 J/kgk
 EXAMPLE 2
EXAMPLE 2
A piece of iron of specific heat capacity,0.04J/KgK and mass 400Kgis quickly dropped into 30kg of water at 100C contained in ac calorimeter of 120Kg mass of C 0.1 J/KgK. If the temperature of the mixture is 30 0C calculate the initial temperature of the hot iron.
SOLUTION
heat lost by hot iron = mi ci (Ǿ3 - Ǿ2 ) = 400 X 0.04 X (Ǿ3 – 30) = 160 Ǿ3- 480
heat gain body = mw cw (Ǿ2 - Ǿ1 ) + mc cc (Ǿ2 - Ǿ1 )
= 30X 4200 X ( 30- 20) + 120 X 0.1 X ( 30-20)
= 2520000 + 240
But heat gain by cold body = heat lost by hot body
2520000 + 240 = 160 Ǿ3- 480
2520000 + 240 + 480 = 160 Ǿ3
Ǿ3 = 2520720/16
Ǿ3 = 157545K
 https://youtu.be/hAbNyM8aKhM
https://youtu.be/hAbNyM8aKhM
SPECIFIC TOPIC: MEASUREMENT OF SPECIFIC HEAT CAPACITY
REFERENCE BOOK: SENIOR SECONDARY PHYSICS,BY OKEKE.
OBJECTIVE: At the end of the lesson, the students should be able to: Enumerate the measurement of specific heat capacity
CONTENT: MEASUREMENT OF SPECIFIC HEAT CAPACITY
Quantity of heat Q is usually obtained using a calorimeter. The most common method used in calorimeter experiment is known as the method of mixtures, another method usually employed is electrical method
TRANSFER OF HEAT
When a hot metallic object is dropped into a copper vessel containing known mass of water at lower temperature, heat continues to pass from the metallic object to the copper vessel and water, until the temperature of the three items (hot metallic object, copper vessel and water) are the same. The hot metallic object loss heat, while the copper vessel and water gain heat. When they finaly come to a steady temperature (same temperature), then we can equate heat lost by hot metallic object to be equal to the heat gained by the copper vessel and water. This is referred as heat transfer.

Photocopiers and Document Reproduction
The photocopiers commonly found in offices and classrooms use electric charges to transfer the image of an original document to a plain piece of paper. The document to be copied is placed face down on the platen and illuminated by a lamp. Its image is directed to a negatively charged metal drum (the electrostatic drum) by a series of mirrors. Where light strikes the drum, the charge disappears, so that dark areas remain charged. Next, positively charged particles of toner powder are brushed onto the drum. These stick only to the charged areas. (The first- and second-erase lamps remove the charge on the drum between different copying tasks.) The image on the drum is then transferred to a piece of paper that has been given a negative charge by the transfer charger. A heater is used to seal the toner to the paper, which is why copies are warm when they emerge.
https://youtu.be/HFxo4j1EWsA
https://youtu.be/NxUbPE8RsiM
MEASUREMENT OF SPECIFIC HEAT CAPACITY OF A SOLID BY METHOD OF MIXTUERS
The apparatus are calorimeter, the stirrer are weighted empty and the weight recorded. Then, the weight of the calorimeter which is then two – thirds filled with water and the stirrer are weighed and recorded, too. The metal block is weighed (mb), the initial temperature of cold water say θ1 is taken. The metal block is put inside a boiling water and the temperature of boiling water is recorded, which is the temperature of the hot metal block say θ2. The metal block is quickly transferred to the lagged calorimeter containing water. The whole content is stirred until all the content come to a final steady temperature. The final temperature of the mixture is taken, say θ f .At this final steady temperature, heat lost by hot metal = heat gained by water and calorimeter.
Hence
mbc(θ2 – θf) = Mccc(θf—θ1) + Mwcw(θf –θ1)
Therefore,
C = Mccc(θf—θ1) + Mwcw(θf –θ1)
Mb(θ2 – θf ) ...............................................................(1.3)
Where c is specific heat capacity of the metal block and Mwcw and McCc are the mass and specific capacity of water and calorimeter respectively.
Consequently heat capacity is mc.
https://youtu.be/LqaThFQyUHI
EVALUATION:
Explain how do we measure the specific capacity of a material
Hot water is added to four times its mass of water at 250C and thoroughly stirred. If the final temperature of the mixture is 400C, calculate the initial temperature of the hot water.
ASSIGNMENT:
.A tap supplies water at 150 C while another supplies water at 900C. If a man wishes to bathe with water at 300C, calculate the ratio of the mass of cold water to the mass of hot water.
EVALUATION
1. A metal of mass 0.5kg is heated to 100ºC, transferred to a well lagged calorimeter of heat capacity 80 J/k containing water of heat capacity 420 J/k at 15ºC. If the final steady temperature of the mixture is 25ºC, find the specific heat capacity of the metal.
3. Heat is supplied to a liquid of mass 500g contained in a can by passing a current of 4A through a heating coil of resistance 12.500 immersed in the liquid. The initial temperature of the liquid is 240C. The liquid reaches its boiling point 10min after the current is switched on, calculate the specific heat capacity of the liquid.
3. Calculate the mass of glass with heat capacity of 670Jkg-1K-1
ASSIGNMENT
1. A body of mass 40g losses 80J of heat. If the specific heat capacity of the body is 400J/KgK. Calculate the change in temperature of the body. (a)0.2K (b)0.4K (c) 2.0K (d)5.0K
2. A 50W electric heater is used to heat a metal block of mass 5Kg. if in 10min a temperature rise of 120C is obtained, the specific heat capacity of the metal is?
(a)130 J/KgK (b) 390 J/KgK (c)400 J/KgK (d) 500J/KgK
3. A water fall 420m is high. Calculate the difference in temperature of the water between the top and bottom of the water fall. Negelect heat loss. [g=10m/s2, specific heat capacity of water= 4200 J/KgK] (a)0.10C (b) 1.0 0C (c) 4.20C (d) 420C
4. A good calorimeter should be (a) low specific heat capacity and low heat conductivity
(b) low specific heat capacity and high heat conductivity
(c) high specific heat capacity and low heat conductivity
(d) high specific heat capacity and low heat conductivity
5. The temperature of a piece of metal of mass 9g is raised from 100C to 1100C when it absorbs 108J of heat energy. Determine the specific heat capacity of the metal in J/KgK (a) 120 (b) (c) (d)
THEORY
1. A copper block of mass 24g at 2300C is placed in a copper calorimeter of mass 60g containing water of mass 54g at 310C,assuming heat loss are negligible, calculate the final steady temperature of the mixture. [specific heat capacity of water= 4200J/KgK specific heat capacity of copper =400J/KgK]
2. A hot body of mass 3g at a temperature θ is transferred into a calorimeter of negligible heat capacity containing water of mass 30g at 15oC. if the mixture is observed to be at 55oC. find the temperature of the hot body. [ specific heat capacity of water 4200J/KgK, specific heat capacity of body= 350 J/KgK]